NICE and NOGG recommendations
NICE 2022 recommendation5
EVENITY is recommended as an option for treating severe osteoporosis in people after menopause who are at high risk of fracture, only if:
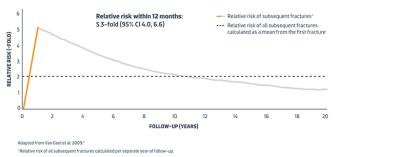
- They have had a major osteoporotic fracture (spine, hip, forearm or humerus fracture) within 24 months (so are at imminent risk of another fracture)
- The company provides EVENITY according to the commercial arrangement
NOGG/ROS Advisory statement on the prioritisation of romosozumab in clinical practice6
Referral for, and consideration of treatment with EVENITY, is prioritised in postmenopausal women who have had a MOF within 24 months, with any one of the following:
- A BMD T-Score ≤-3.5 (at the hip or spine)
- A BMD T-score ≤-2.5 (at the hip or spine
- Vertebral fractures (either a vertebral fracture within 24 months or a history of ≥2 osteoporotic vertebral fractures)
- Very high fracture risk (e.g., as quantified by FRAX)
Following the approved duration of treatment with EVENITY (12 months), treatment with alendronate, zoledronate or denosumab should be initiated without delay.
EVENITY is indicated in treatment of severe osteoporosis in postmenopausal women at high risk of fracture1
EVENITY is contraindicated in patients with hypersensitivity, hypocalcaemia and history of myocardial infarction or stroke.1
What’s behind EVENITY
From the frontiers of space to the front line of fracture treatment. Watch our short film to discover exactly how EVENITY became reality.